Our solutions
Our customers

Sallea, a Swiss startup founded in 2023, specializes in edible macro scaffolds enabling the production of large, texturized cultivated meat. Their vision centers on becoming the leading technology provider enabling texturized, cultivated products that accelerate the transition to more sustainable animal protein production.
As a scaffold provider, sallea must support its cultivated meat customers' transition to commercial food production. Pharmaceutical-Grade DMEM-F12 remains unsuitable for large-scale food applications due to Non-Food-Grade components and high costs that create barriers for commercial scaling. They need media solutions enabling robust cell attachment on protein scaffolds while meeting food safety standards for end customers. They also require cost-effective FBS alternatives that eliminate batch variability and align with sustainability goals without compromising performance.
Sallea evaluated Multus Food-Grade DMEM-F12 to demonstrate biocompatibility with their scaffold platform for structured cultivated meat production. Testing focused on compatibility between two products from Sallea's ProteinPURE scaffold line, PP25 (pea-protein based) and SP25 (soy-protein based) scaffolds, using both model and food-relevant cells. Cells resuspended in Multus Food-Grade DMEM-F12 with FBS (90%-10%) were seeded on scaffolds that were previously coated with 100% FBS. Cell performance was assessed through MTT metabolic activity assays at day 0 (d0, attachment) and day 4 (d4). MTT assays measure cellular metabolic activity through reduction of yellow tetrazolium salt to purple formazan crystals by mitochondrial dehydrogenases. Higher absorbance values (600 nm/gr scaffold) indicate greater metabolic activity and are used as a proxy for cell number and health. Data was normalized to scaffold weight after one-hour attachment (d0) or four days of culture (d4).
Using our AI-driven platform, we developed Multus Food-Grade DMEM-F12 to achieve Pharmaceutical-Grade cell culture performance using Food-Grade ingredients. Manufactured in an FSSC 22000-certified facility, Multus Food-Grade DMEM-F12 provides regulatory-compliant media formulations essential for commercial cultivated meat production.
Sallea's comparative analysis reveales significant advantages of using sallea’s ProteinPURE scaffolds with Multus Food-Grade DMEM-F12 compared to a common, Pharmaceutical-Grade DMEM-F12 product:
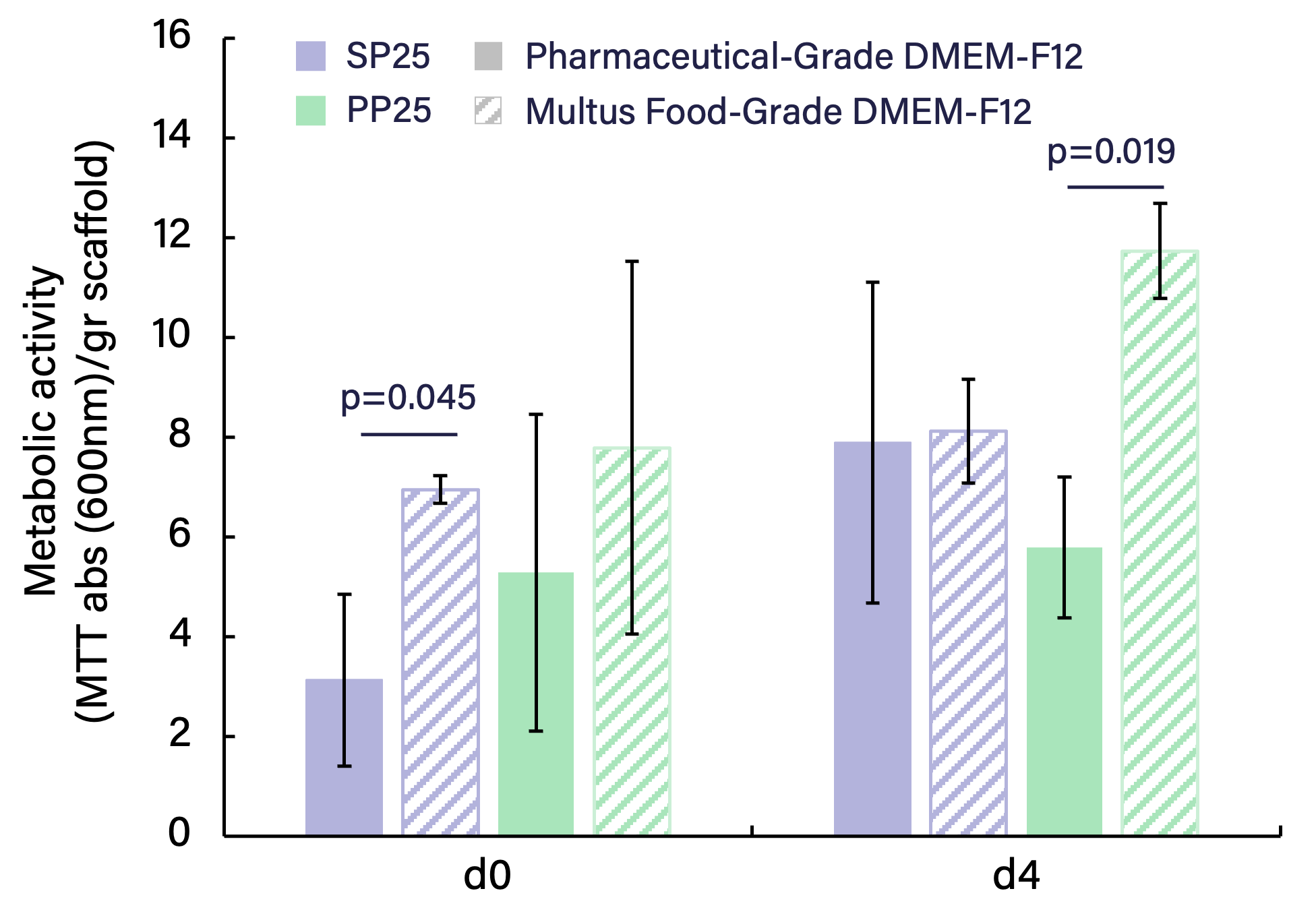

Building on the successful Multus Food-Grade DMEM-F12 results, sallea is expanding testing with Proliferum B to replace FBS coating and create a fully animal component-free system that meets their customers' regulatory requirements. Initial tests combining Proliferum B with their model cell line have shown promising results, and sallea is currently expanding these studies to include primary bovine satellite cells and longer culture periods to validate the scaffold-media system.
"The combination of our scaffold platform with Multus Food-Grade DMEM-F12 paves a clear path for our customers to overcome key regulatory hurdles in commercial meat production. Integrating Proliferum B as an animal-component-free alternative to FBS is an even bigger leap forward. With these promising results, we're excited to deepen our collaboration with Multus to optimize their products for our platform, unlocking the full potential of cellular growth and delivering exceptional solutions for our customers." – Simona Fehlmann, CEO and Co-Founder of Sallea
By enabling robust cell attachment on edible scaffolds with Food-Grade basal media and serum-free alternatives, Multus Food-Grade DMEM-F12 combined with Proliferum B will provide cultivated meat companies with a clear regulatory pathway from research to commercial production, supporting the industry's transition toward sustainable, structured meat products that meet both performance and compliance requirements at scale.
___________
Technical Note: Multus Food-Grade DMEM-F12 was formulated using Multus’ AI-driven platform that systematically replaced every Non-Food-Grade component with Food-Grade analogues. Cell performance was uncompromised during development, demonstrated by superior cell adhesion and expansion compared to Pharmaceutical-Grade DMEM-F12.
Sign up for email updates: