
Advanced cell and gene therapies require specialized media for novel cell types: from stem cells and NK cells to AAV production and CAR-T manufacturing. Traditional media development takes 2-4 years and costs millions of dollars per formulation, creating a critical barrier to manufacturing scale-up and patient access. These prohibitive costs and timelines prevent breakthrough therapies from reaching commercial scale and the patients who need them.
Our MediOPTM platform accelerates media development for the diverse cell types used in advanced therapies, from early-stage research through commercial manufacturing. We help you overcome the cost and timeline barriers that prevent therapeutic innovations from reaching patients.
Custom media development tailored to your therapeutic cell type. From rapid Express screening and Boost optimization of existing formulations to complete De Novo design, our platform addresses the specific challenges of stem cells, immune cells, and viral production systems.
Learn more

Seamless transition from optimized formulation to GMP-like production. Our global partner network ensures you can access therapeutic-grade media at clinical and commercial scales.
Learn more
Formulations tailored to your therapeutic cell type's unique requirements, optimized for viability, potency, and consistency in manufacturing.
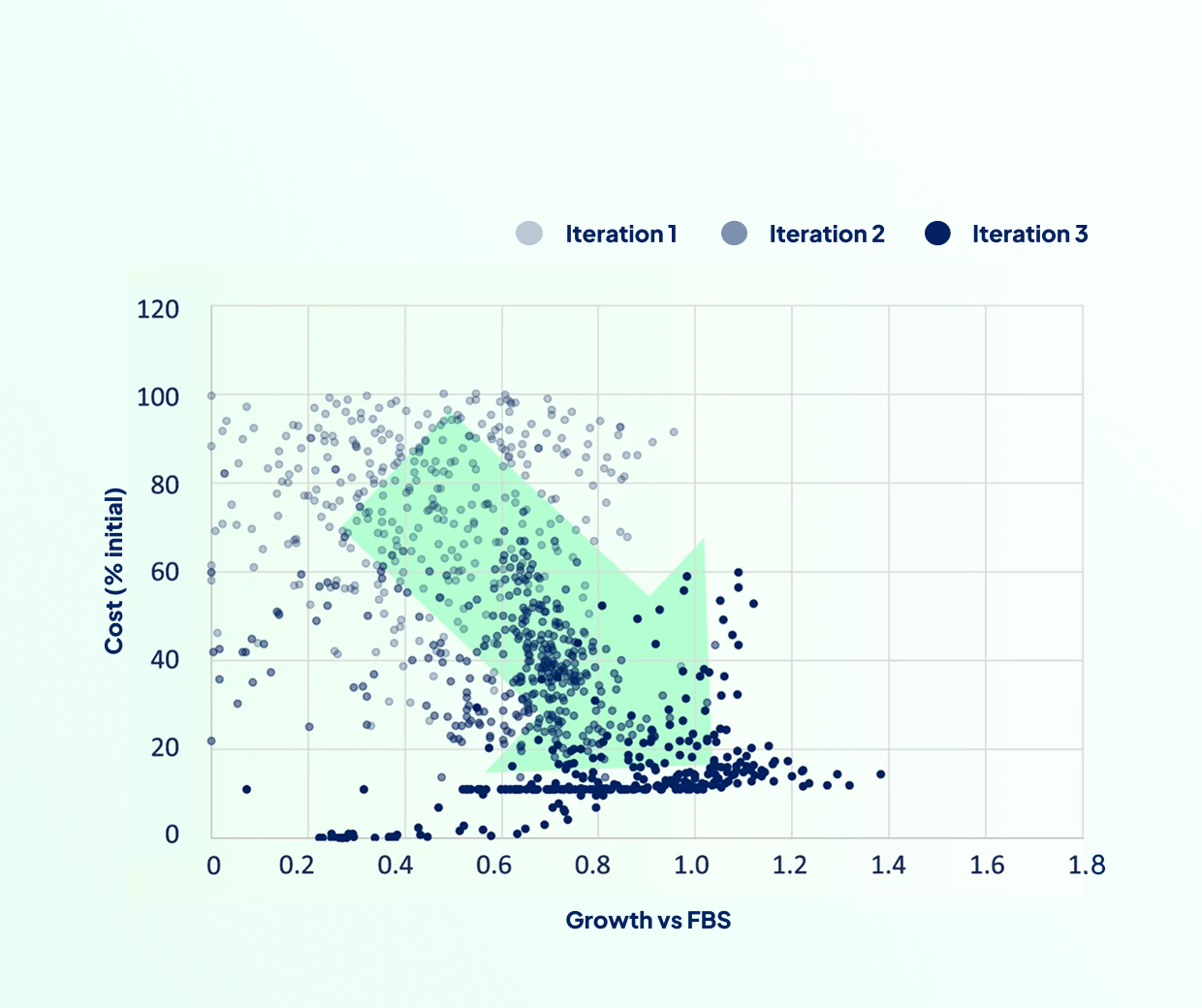
Deliver optimized media in weeks instead of years, at a fraction of traditional costs.

Flexible development pathways from catalog screening to complete custom design, matching your development stage and clinical timeline requirements.


















No other team is as dedicated to advancing cell culture media as Multus. Talk to our media specialists today!
